Cytopoint: Making a Difference for Dogs with Atopic Dermatitis
Canine atopic dermatitis has been defined as “a genetically-predisposed inflammatory and pruritic allergic skin disease with characteristic clinical features associated with IgE antibodies most commonly directed to environmental allergens”. Itching, scratching, rubbing, licking, a yeasty smell, redness, tough or greasy skin are the most common symptoms.
The treatment of canine AD is composed of multiple interventions with the intent to grasp the whole scope of the disease. Additionally, ingestion of food allergens or insect bites appears to worsen signs of AD hence accentuating the role of a proper dog diet and preventive measures. Various drugs with anti-inflammatory or antipruritic effects are the first method of attack by many veterinary practitioners. When microbial allergens are the suspected culprits for the exacerbation of this condition, antibacterial or antifungal medications can be of great use.
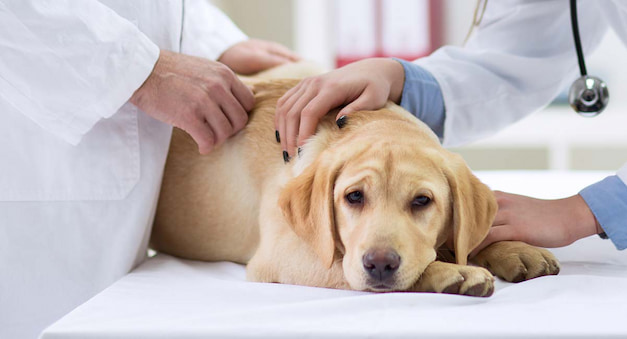
Most recently, allergen-specific immunotherapy (ASIT) like CADI for dogs has been taking over the front in the battle against IgE-activating environmental antigens i.e. used to prevent relapses of canine AD in future allergen exposure.
What Is Cytopoint & How Does it Work?
A slowly increasing number of studies in dogs have documented the effectiveness of ASIT with allergic diseases. ASIT is the only current treatment for allergy that can not only change but also reverse, at least partly, the pathogenesis of this condition. Luckily, getting CADI for dogs can alleviate your dog’s clinical signs and prevent the progression of the disease.
The mechanisms by which ASIT works in dogs have not been completely elucidated, although they are likely to parallel those known in humans: early reduction in effector cell activity (eosinophils, basophils, mast cells) followed by a long-term immunologic shift from a T helper 2 (Th2) cell to a T helper 1 (Th1) cell response and development of immunological tolerance.
This modification is carried out without the possible long-term adverse effects of a lifetime of drug treatment, with minimal adverse effects, and with the potential of long-lasting effectiveness.
What Is the Active Ingredient in Cytopoint?
The active compound in Cytopoint is Lokivetmab. Its advantages are its preciseness and a very long half-life, keeping its concentration in the circulation adequate for several weeks. Even so, repeated administration, monthly or as needed, is allowed.
Lokivetmab is a monoclonal antibody (mAb) that specifically binds only canine interleukin 31 (IL-31). IL-31 is a T-cell derived proinflammatory cytokine of the IL-6 family of cytokines. Monoclonal antibodies, on the other hand, are complex large protein molecules that are developed by using recombinant DNA technology. They are designed to mimic or evoke the natural immune response in the body. They’re given by injection, not orally, as they are inactivated by digestion.
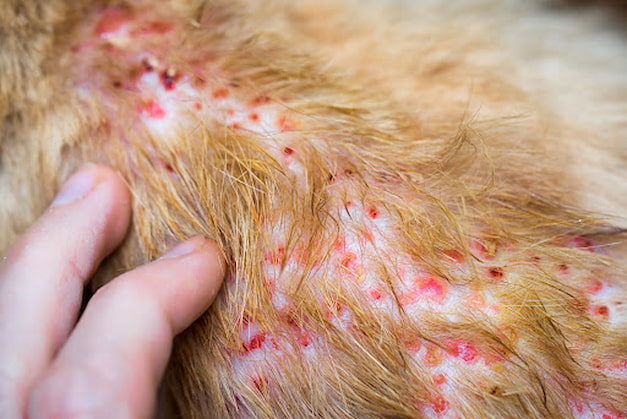
IL-31 is mainly produced by TH2 cells. It has a substantial role in innate and adaptive immunity and is most abundant in tissues that are in close and continuous contact with the environment, i.e. the skin, the airways and the lung. In spite of its protective role, IL-31 is also associated with a number of diseases, including atopic dermatitis (AD), when dysregulation of the immune cells occurs. Interestingly, there’s no clinical disease attributed to the absence of IL-31 signalling, only due to its excess.
In addition to Th2 cells, other cells that produce IL-31 include the rest of the T-cells (CD4+, CD8+, TH1), macrophages, dendritic cells, mast cells, keratinocytes, and fibroblasts. These cell types are linked through several mechanisms with IL-31, itching (pruritus) and atopic dermatitis. Cells that express the IL-31 receptor include monocytes, eosinophils, mast cells, dendritic cells, keratinocytes, human dermal microvascular endothelial cells, primary cells of bronchial epithelia,
Cytopoint may be effective in some dogs with an insufficient response to first forms of allergy therapy, including cases that have failed oclacitinib therapy. One clinical trial proved a single injection of 2 mg/kg effective at reducing pruritus within 1 day. Also, it was effective for a full month.

On day 3, more than 80% of dogs administered Cytopoint achieved treatment success predefined by pruritus scores. Moreover, the efficacy was substantially bigger compared to placebo in achieving treatment success in the improvement of skin lesion scores. Overall significant improvement in this skin condition was noted at the first visit on day 7. The side effects were minimal, manageable and, interestingly, similar to the placebo.
What Are the Side Effects of Cytopoint?
One of the advantages of Cytopoint is its safety, for dogs of all ages or ones using concomitant medication. CADI for dogs, being an antibody protein, it’s naturally broken down by and recycled by the body. Adverse effects are not expected, except for possible tolerance after a few treatments.
Vomiting, diarrhea and lethargy have been reported. Even so, one study showed that Cytopoint was well tolerated in dogs after subcutaneous injection. Once again, adverse events occurred at a similar frequency between treated and placebo groups, only this time the study included 245 canine patients. There were no other health events throughout the study that weren’t self-limiting. In addition, conjunct therapy was not interrupted and a variety of concomitant medications were safely used.



